Explain This Difference in Boiling Points Chegg
Assume the air temperature is 100 C and that Δ v a p H 41 k J m o l 1 H 2 O. The formula for boiling point elevation is.
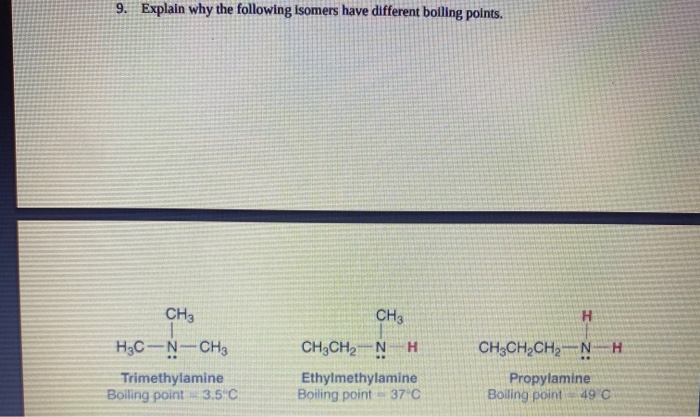
Solved 9 Explain Why The Following Isomers Have Different Chegg Com
Check Your Learning Ethane CH 3 CH 3 has a melting point of 183 C and a boiling point of 89 C.

. Chemistry questions and answers. P P 0 10 M g h 2303 R T where P pressure in atm. Explain the trend in boiling points from H2S to H2Te.
Acceleration due to gravity g 981 m s 2. Non-electrolytes dont dissociate when they dissolve. Explain why the third ionization energy of.
Chemical X has a boiling point of 75 oC Chemical Y has a boiling point of 126 oC 23. Boiling Article What is Evaporation. -269 C -452 F.
Explain the difference in boiling points between the members of the following pairs of substances. Predict the melting and boiling points for methylamine CH 3 NH 2. A 211 mixture of 2-chloro-2-methylpropane MW.
Molar mass of air M 002896 k g m o l 1. ΔT b iKbm. We review their content and use your feedback to keep the quality high.
Explain the difference in boiling points based on what you know about intermolecular forces. Explain this difference in boiling points in terms of intermolecular forces. Experts are tested by Chegg as specialists in their subject area.
Evaporation is a process where liquid turn into vapor. 7837 C 1731 F Boiling point of nitrogen. Boiling point of water.
Explain the differences in boiling point of a 1-pentene bp 30 degree C 129 degree C 1-bromopentane bp 129 degree C and 1-pentanol bp 137 degree C. Discuss the difference between the ionic and covalent compounds with respect to the boiling and melting point of the compound. Between two nonpolar molecules of similar mass the more extended molecule will have the higher boiling point more extended à more surface area for London dispersion interaction.
Explain this difference in boiling points in terms of heats of vaporization. Bp 133 degree C. CH 3CH 2CH 3 CH 3CH 2OH bp 42 C bp 78 C 2.
R 83145 J m o l 1 K 1. Explain the difference in their boiling points. Example is water evaporated from the soil What is Boiling.
1-butanol has the higher boiling point because the molecules can form hydrogen bonds with each other It contains an OH bond. Why do the boiling points of the noble gases increase in the order He Ne Ar Kr Xe. 100 C 212 F Boiling point of water in Kelvin.
It happens when a liquid is heated to its boiling point. The boiling occurs in three different stages such as. Distillation is a physical separation method that is used to separate compounds from a.
And T is the Kelvin temperature. 647 C 1485 F Boiling point of acetone. 7837 C 1731 F Boiling point of methanol.
Explain the differences in boiling point of a 1-pentene bp 30C 1-bromopentane bp 129C and 1-pentanol bp 137C. Bp 85 degree C. P 0 1 atm.
Therefore these molecules will be liquids at 25 25. Chemists will have to come up with rational explanations for these deviations based on the chemical natures of compounds C and A. A HF 20C and HCI -85C b CHCI 61C and CHBr 150C c Br59C and ICI 97C.
The melting and boiling points of propane are both below 25 25 therefore the molecule will be a gas at 25 25. Explain this difference in conductivity in terms of the structures of sodium and sodium iodide. Diethyl ether has two polar C.
What intermolecular forces are involved in holding the molecules in the liquid form in each of these as pure substances. Explain the difference in boiling points between the members of the following pairs of substances. Predict the major organic products of the following reactions.
The London forces typically increase as the number of electrons increase. Refer to the graph on the right to answer the following questions. -1958 C -3204 F Boiling point of liquid helium.
The melting points of butanoic acid bromoethane and dimethyl ether are below 25 25 however the boiling points of all three molecules are above 25 25. On the basis of intermolecular attractions explain the differences in the boiling points of n-butane 1 C and chloroethane 12 C which have similar molar masses. Diethyl ether and 1-butanol are similar in size number of electrons therefore their boiling points will be determined by polarity.
Electrovalent or ionic compounds have a high boiling and melting point whereas covalent compounds have a low melting and boiling point. Where m is the molality of the solution Kb is the molal boiling point elevation constant for the solvent and i is a number related to the number of particles the solute contributes to the solution the vant Hoff i factor. Diethyl ether molecules do contain both oxygen atoms and hydrogen atoms.
Explain the difference in boiling point between the isomers 1-chlorobutane and 2-methyi- 2-chloropropane in terms of structure. Intermolecular force has a higher boiling point Look for functional groups that may indicate polar molecule. 56 C 1328 F Boiling point of alcohol.
Which substance would be expected to have the highest boiling point. However we see that compound C has a higher boiling point than the trend would predict for it s molar mass and compound A has a lower boiling point that the trend would predict for its molar mass. 3732 K Boiling point of ethanol.
The key difference between fractional and simple distillation is that fractional distillation is used w hen the components in the mixture have closer boiling points while simple distillation is used when the components in the mixture have a large difference in their boiling points. A HF 20C and HC -85C b Bra 59C and ICI 97C 3. Difference Between Evaporation and Boiling Evaporation vs.
The N2 has the higher boiling point because it has greater dispersion forces. Boiling means rapid vaporization of any liquid. The boiling point of propane is 421 C the boiling point of dimethylether is 248 C and the boiling point of ethanol is 785 C.
Chemical X has weaker intermolecular forces than Chemical Y 24. What intermolecular force are involved in holding the molecules in the liquid form in each of these as pure substances. But only sodium can conduct electricity when solid.
Ad Get 247 access to step-by-step textbook solutions and expert QA. The chemical element with the lowest boiling point is Helium and the element with the highest boiling point is Tungsten.

Solved Explain Why There Is A Difference Between The Boiling Chegg Com
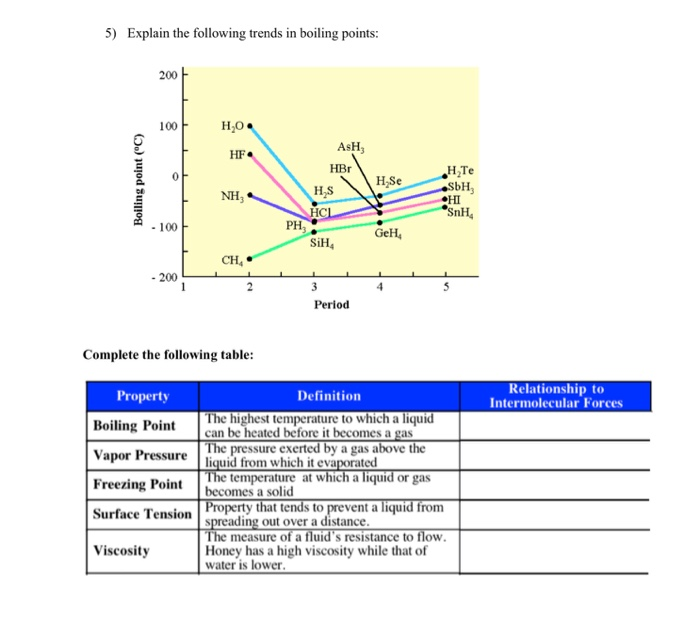
Solved 5 Explain The Following Trends In Boiling Points Chegg Com
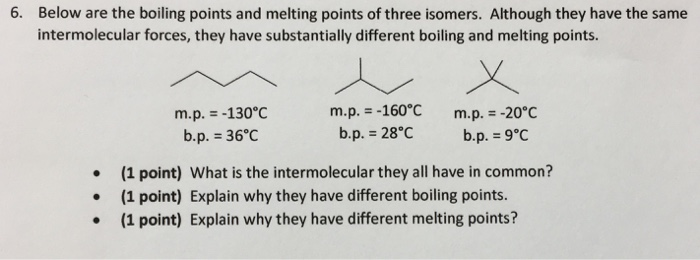
Solved 6 Below Are The Boiling Points And Melting Points Of Chegg Com
No comments for "Explain This Difference in Boiling Points Chegg"
Post a Comment